In this article, we dive into melanoma and how researchers are using the immune system to target this cancer.
Growing Pains: Why are Blood and Brain Cancers Dominant in Children?
In this post, learn more about the biology behind why blood and brain cancers commonly affect children.
A Spotlight on Head and Neck Cancer
This past April was Head and Neck Cancer Awareness Month and RIOT dived into the subject to talk about the latest research on the disease. Learn more look here!
M x M x M x M = M⁴
RIOT highlights Multiple Myeloma Awareness Month in this blog post. Stay informed on the development and current treatments of multiple myeloma, a cancer that forms in white blood cells.
Biliary Tract Cancers: A Spotlight on a Rare Group of Diseases
Check out this blog post to learn more about biliary cancers - a rare group of cancers of the biliary system, which include the gallbladder and bile duct
ATTENTION READERS, GET A PAP TEST!
RIOT highlights Cervical Cancer Awareness Month in this blog post. Stay informed on cervical cancer and the Pap test - a key screening technique to help detect this disease.
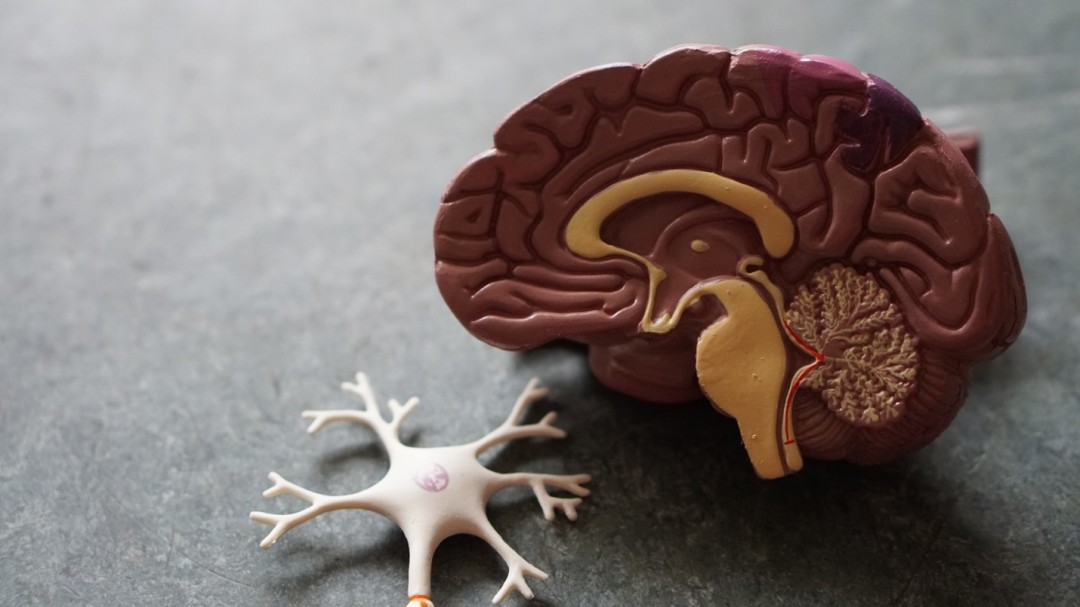
Brain Cancer Cells Grow by Tapping into Host Brain Cell Signals
In this article we dive into gliomas - a difficult to treat brain cancer - by exploring the unique way neurons communicate. Understanding this phenomenon may be the key to unlocking better treatments.
COVID, Cancer, and the Immune System
COVID-19 is all over the news. What does this mean for cancer patients? Take a look at this article to learn more about the inner workings of our immune system and the importance of keeping those who have a compromised immune system safe during these times.
CRISPR: Answering your questions on the gene editing technology everybody is talking about
A deep dive into the science and implications of CRISPR technology.
Cancer cells are stressed out and it may be a good thing
DNA in our cells gets damaged everyday. Our cells can also makes mistakes when replicating our DNA. These mistakes and damages are known as "replication stress." Cancer cells are unique because they have chronic replication stress and use it to their advantage. Check out this blog article to learn more about this phenomenon and how scientists are trying to exploit it to help treat cancer.